“
The intricate communication system of the body relies on action potentials and ion channels, which form the foundation of nerve signal transmission. These electrical impulses and protein gateways maintain brain functions, control muscles, and regulate sensations. Ion channels manage the flow of charged atoms like sodium and potassium, while action potentials ensure quick, targeted communication across nerve cells.1
1
”
Ancient physician Galen once described nerve function as “pneuma flow,” unaware that what he called “spirit” was a precursor to today’s understanding of action potentials and ion channels guiding electric signals. 1
Action potentials begin when a neuron’s membrane potential reaches a critical threshold, causing voltage-gated sodium ion channels to open, allowing positive sodium ions to rapidly enter the neuron’s interior. 2
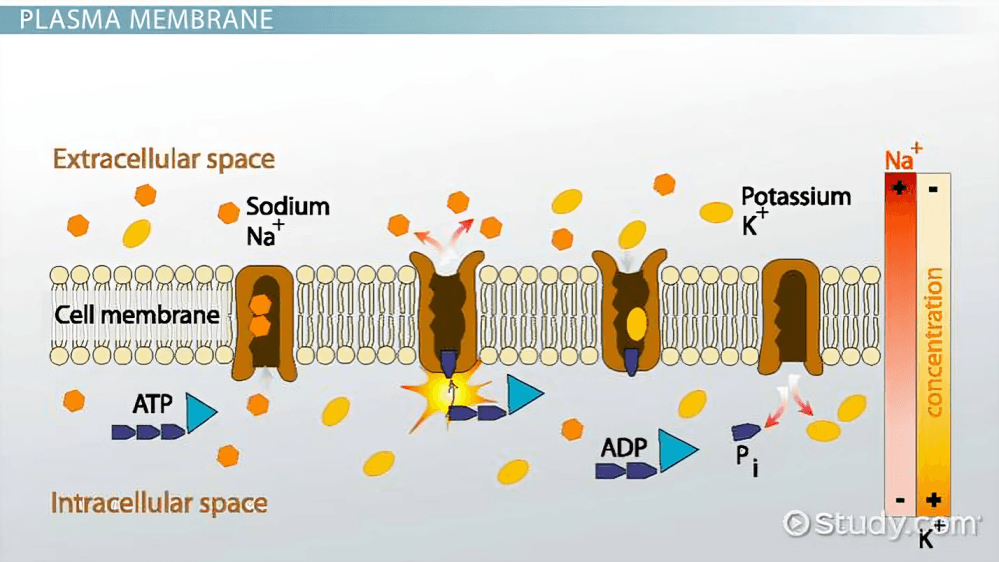
After sodium influx, potassium ion channels open, allowing potassium ions to exit the neuron, which restores the internal negative charge in a process called repolarization—a crucial reset before another impulse.
The sodium-potassium pump, an essential protein in the membrane, restores the original ion distribution by pumping out sodium and bringing in potassium after every action potential, maintaining neuron readiness. 3
Myelinated axons use saltatory conduction, where action potentials leap from one node of Ranvier to the next, significantly speeding up signal transmission compared to unmyelinated nerve fibers. 4
Ion channels are selective, only allowing specific ions like Na⁺, K⁺, Ca²⁺, or Cl⁻ to pass, depending on the channel type—this selectivity is vital for accurate neuronal signaling and muscle control. 5
Voltage-gated channels open in response to changes in membrane voltage, while ligand-gated channels respond to chemical messengers—both play distinct roles in initiating or modifying action potentials. 6
Ion channels can become inactivated shortly after opening, ensuring action potentials travel in only one direction along the axon and preventing backtracking of the electrical impulse. 7
A neuron’s refractory period follows each action potential, during which it cannot fire again. This resting phase ensures orderly signal propagation and prevents signal overlap or chaos. 8
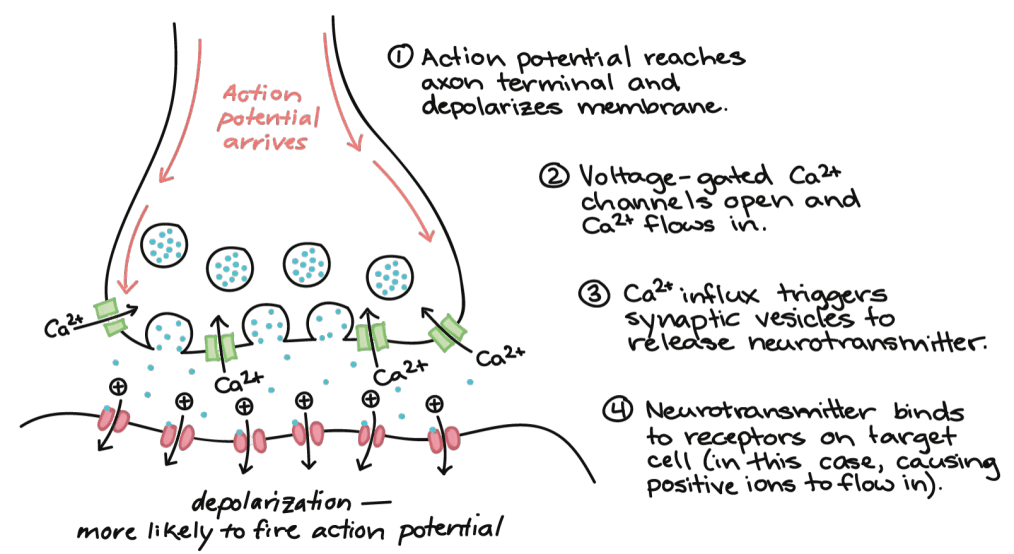
Calcium ion channels play a key role in neurotransmitter release at synapses. When an action potential reaches the axon terminal, calcium enters, triggering the release of signaling molecules.
Neurotoxins like tetrodotoxin block voltage-gated sodium channels, preventing action potentials entirely, which leads to paralysis or death, showing how crucial these channels are to survival. 9
Different types of neurons have different firing patterns. Some fire rapidly in bursts, while others send signals more slowly, all governed by the properties of their ion channels and internal thresholds. 10
The all-or-nothing principle means that an action potential either happens fully or not at all; once the threshold is hit, a complete action potential follows with the same intensity each time. 11
During the depolarization phase, the neuron’s inside becomes less negative and even positive compared to the outside, driven primarily by sodium ion entry through voltage-gated channels. 12
Ion channel blockers are used medically to manage pain, epilepsy, hypertension, and arrhythmias by controlling the activity of specific channels and thus modifying the generation of action potentials. 13
Sensory neurons rely on mechanically gated ion channels that respond to touch, pressure, or sound, converting external stimuli into electrical signals processed by the brain via action potentials. 14
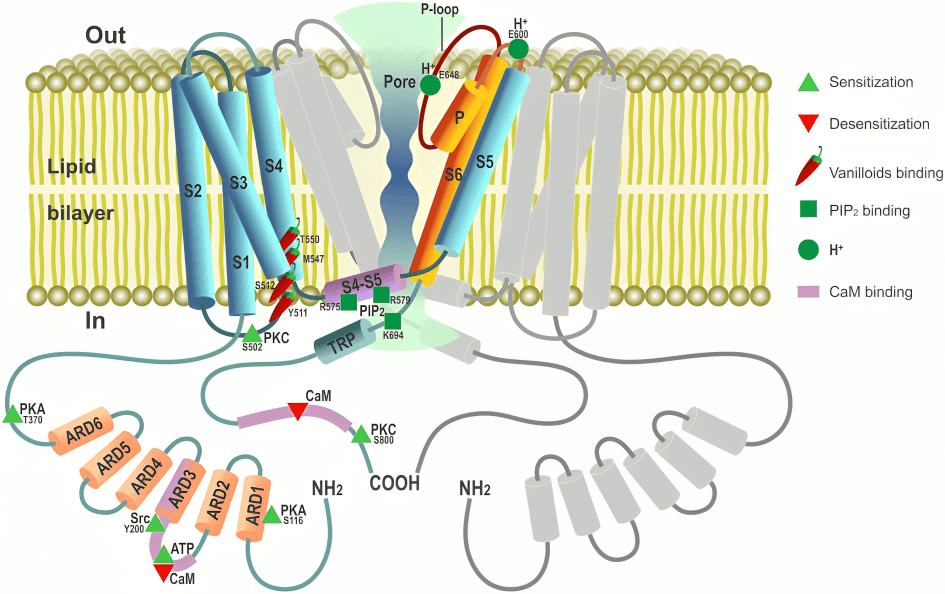
Temperature-sensitive ion channels like TRPV1 open in response to heat or spicy compounds like capsaicin, triggering nerve signals that the brain interprets as pain or burning sensations.
Some ion channels are regulated by intracellular signals like cyclic AMP or calcium levels, allowing the neuron to adjust its response based on internal cellular conditions and ongoing activity. 15
Some drugs act as channel openers, enhancing ion flow to increase nerve excitability in certain therapeutic cases, such as spinal cord injuries or neurodegenerative diseases like multiple sclerosis. 16
Ion channels are not static; they undergo conformational changes, opening or closing their gates based on voltage, binding chemicals, or mechanical stretch, acting as dynamic sensors of cellular environments. 17


